Highlights
Glucose Oxidase Mediated Gelation: A Simple Test to Detect Glucose in Food Products
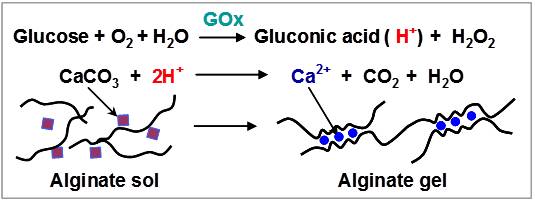 We report a simple, rapid and sugar-selective method to induce gelation from glucose-containing samples. This method employs glucose oxidase (GOx) to selectively “recognize” and oxidize glucose to generate gluconic acid, which acts to solubilize calcium carbonate and release calcium ions. The release of calcium ions triggers gelation of the calcium-responsive polysaccharide alginate to form a calcium-alginate hydrogel. Rheological measurements confirm that gel-formation is triggered by glucose but not fructose or sucrose (consistent with GOx’s selectivity). Vial inversion tests demonstrate that gel-formation can be readily observed without the need for instrumentation. In proof-of-concept studies, we demonstrate that this gel-forming method can detect glucose in food/beverage products sweetened with glucose or high fructose corn syrups. These results indicate that the enzyme-induced gelation of alginate may provide a simple means to test for sweeteners using components that are safe for use on-site or in the home. (J. Agric. Food Chem. 2012, 60, 8963-8967. DOI:10.1021/jf301376b).
We report a simple, rapid and sugar-selective method to induce gelation from glucose-containing samples. This method employs glucose oxidase (GOx) to selectively “recognize” and oxidize glucose to generate gluconic acid, which acts to solubilize calcium carbonate and release calcium ions. The release of calcium ions triggers gelation of the calcium-responsive polysaccharide alginate to form a calcium-alginate hydrogel. Rheological measurements confirm that gel-formation is triggered by glucose but not fructose or sucrose (consistent with GOx’s selectivity). Vial inversion tests demonstrate that gel-formation can be readily observed without the need for instrumentation. In proof-of-concept studies, we demonstrate that this gel-forming method can detect glucose in food/beverage products sweetened with glucose or high fructose corn syrups. These results indicate that the enzyme-induced gelation of alginate may provide a simple means to test for sweeteners using components that are safe for use on-site or in the home. (J. Agric. Food Chem. 2012, 60, 8963-8967. DOI:10.1021/jf301376b).
Biofabricating Multifunctional Soft Matter with Enzymes and Stimuli-Responsive Materials
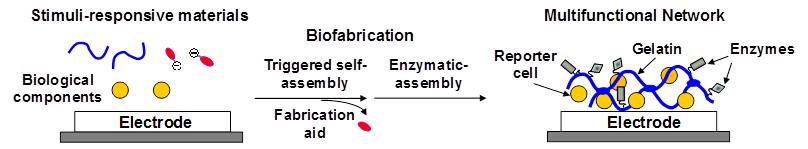 A multifunctional matrix is biofabricated by coupling self-assembly with enzymatic-assembly. Self-assembly is triggered using stimuli-responsive materials and device-imposed electrical signals to electrodeposit a gelatin matrix. Enzymatic reactions serve to crosslink the gelatin and conjugate proteins to this matrix. These studies demonstrate the potential for enlisting biological materials and mechanisms to construct multifunctional soft matter. (Adv. Funct. Mater. 2012, 22, 3004-3012. DOI:10.1002/adfm.201200095).
A multifunctional matrix is biofabricated by coupling self-assembly with enzymatic-assembly. Self-assembly is triggered using stimuli-responsive materials and device-imposed electrical signals to electrodeposit a gelatin matrix. Enzymatic reactions serve to crosslink the gelatin and conjugate proteins to this matrix. These studies demonstrate the potential for enlisting biological materials and mechanisms to construct multifunctional soft matter. (Adv. Funct. Mater. 2012, 22, 3004-3012. DOI:10.1002/adfm.201200095).
Biofabrication: Programmable Assembly of Polysaccharide Hydrogels in Microfluidics as Biocompatible Scaffolds
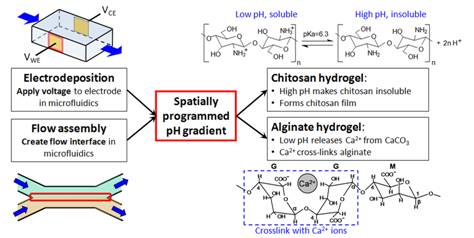 Because of their stimuli-responsiveness to chemical and pH gradients, polysaccharide hydrogels such as chitosan and alginate can be assembled as scaffolds for biomolecules or cells. Using the electrical and flow control available in microfluidic networks, in situ fabrication of 3D hydrogel scaffolds can be programmed in space and time to arrange biological components as an in vitro biochemically communicating system. Flexible in situ on-demand construction of a biocompatible scaffold within microfluidics holds promise for the assembly of biological components and systems for in vitro analysis and investigation. We foresee a wide spectrum applications ranging from replication of metabolic pathways as testbeds for drug discovery to identification of cell signaling mechanisms and observation of cellular response. (J. Mater. Chem. 2012, 22, 7659-7666. DOI: 10.1039/c2jm16215f).
Because of their stimuli-responsiveness to chemical and pH gradients, polysaccharide hydrogels such as chitosan and alginate can be assembled as scaffolds for biomolecules or cells. Using the electrical and flow control available in microfluidic networks, in situ fabrication of 3D hydrogel scaffolds can be programmed in space and time to arrange biological components as an in vitro biochemically communicating system. Flexible in situ on-demand construction of a biocompatible scaffold within microfluidics holds promise for the assembly of biological components and systems for in vitro analysis and investigation. We foresee a wide spectrum applications ranging from replication of metabolic pathways as testbeds for drug discovery to identification of cell signaling mechanisms and observation of cellular response. (J. Mater. Chem. 2012, 22, 7659-7666. DOI: 10.1039/c2jm16215f).
Electrodeposition of a Biopolymeric Hydrogel: Potential for One-step Protein Electroaddressing
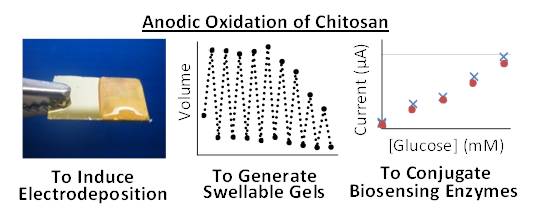 The electrodeposition of hydrogels provides a programmable means to assemble soft matter for various technological applications. We report an anodic method to deposit hydrogel films of the aminopolysaccharide chitosan. Evidence suggests the deposition mechanism involves the electrolysis of chloride to generate reactive chlorine species (e.g., HOCl) that partially-oxidize chitosan to generate aldehydes that can couple covalently with amines (presumably through Schiff base linkages). Chitosan’s anodic deposition is controllable spatially and temporally. Consistent with a covalent crosslinking mechanism, the deposited chitosan undergoes repeated swelling/de-swelling in response to pH changes. Consistent with a covalent conjugation mechanism, proteins could be co-deposited and retained within the chitosan film even after detergent washing. As a proof-of-concept, we electroaddressed glucose oxidase to a side-wall electrode of a microfabricated fluidic channel and demonstrated this enzyme could perform electrochemical biosensing functions. Thus, anodic chitosan deposition provides a reagentless, single-step method to electroaddress a stimuli-responsive and biofunctionalized hydrogel film. (Biomacromolecules, 2012, 13, 1181–1189. DOI: 10.1021/bm3001155).
The electrodeposition of hydrogels provides a programmable means to assemble soft matter for various technological applications. We report an anodic method to deposit hydrogel films of the aminopolysaccharide chitosan. Evidence suggests the deposition mechanism involves the electrolysis of chloride to generate reactive chlorine species (e.g., HOCl) that partially-oxidize chitosan to generate aldehydes that can couple covalently with amines (presumably through Schiff base linkages). Chitosan’s anodic deposition is controllable spatially and temporally. Consistent with a covalent crosslinking mechanism, the deposited chitosan undergoes repeated swelling/de-swelling in response to pH changes. Consistent with a covalent conjugation mechanism, proteins could be co-deposited and retained within the chitosan film even after detergent washing. As a proof-of-concept, we electroaddressed glucose oxidase to a side-wall electrode of a microfabricated fluidic channel and demonstrated this enzyme could perform electrochemical biosensing functions. Thus, anodic chitosan deposition provides a reagentless, single-step method to electroaddress a stimuli-responsive and biofunctionalized hydrogel film. (Biomacromolecules, 2012, 13, 1181–1189. DOI: 10.1021/bm3001155).
Electroaddressing Functionalized Polysaccharides as Model Biofilms for Interrogating Cell Signaling
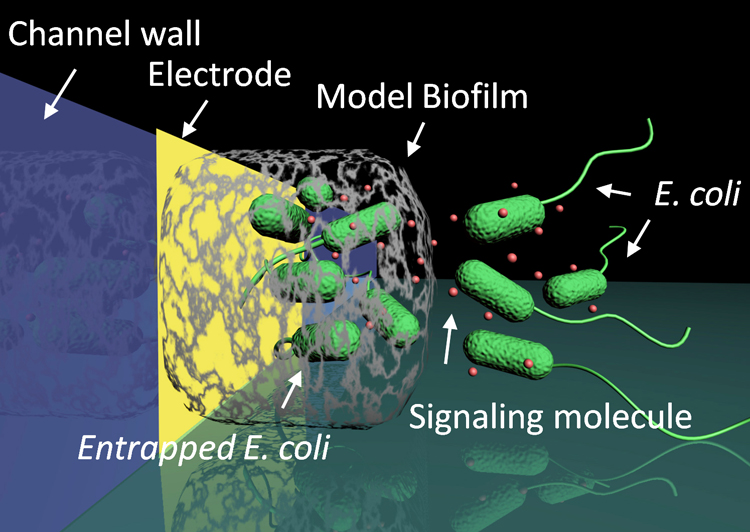 The functionality of stimuli-responsive polysaccharide alginate is demonstrated by biofabricating 3D cell-gel biocomposites, mimicking the formation of biofilms, for interrogating phenotypes of E. coli bacterial populations. By using device-imposed electrical signals, the bacterial population density and distribution can be spatiotemporally controlled. This approach can be exploited for investigating biofilm development and bacterial intra- and interspecies signaling in controlled patterns. (Adv. Func. Mater. 2012, 22, 519-528. DOI: 10.1002/adfm.201101963).
The functionality of stimuli-responsive polysaccharide alginate is demonstrated by biofabricating 3D cell-gel biocomposites, mimicking the formation of biofilms, for interrogating phenotypes of E. coli bacterial populations. By using device-imposed electrical signals, the bacterial population density and distribution can be spatiotemporally controlled. This approach can be exploited for investigating biofilm development and bacterial intra- and interspecies signaling in controlled patterns. (Adv. Func. Mater. 2012, 22, 519-528. DOI: 10.1002/adfm.201101963).
Reversible Electroaddressing of Self-Assembling Amino Acid Conjugate
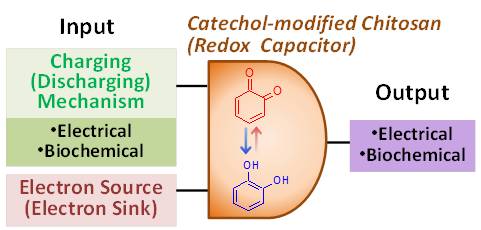 Electronic devices process information and transduce energy with electrons, while biology performs such operations with ions and chemicals. To establish bio-device connectivity, we fabricate a redox-capacitor film from a polysaccharide (i.e., chitosan) and a redox-active catechol. We report that these films are rapidly and repeatedly charged and discharged electrochemically via a redox-cycling mechanism in which mediators shuttle electrons between the electrode and film (capacitance ˜ 40 F/g or 2.9 mF/cm2). Further, charging and discharging can be executed under bio-relevant conditions. Enzymatic-charging is achieved by electron-transfer from glucose to the film via an NADPH-mediated redox-cycling mechanism. Discharging occurs by electron-donation to O2 to generate H2O2 that serves as substrate for peroxidase-mediated biochemical reactions. Thus, these films offer the capability of inter-converting electrochemical and biochemical inputs/outputs. Among potential applications, we anticipate that catechol–chitosan redox-capacitor films could serve as circuit elements for molecular logic operations or for transducing bio-based chemical energy into electricity. (Adv. Funct. Mater. 2012, 22, 1409–1416. DOI: 10.1002/adfm.201101946).
Electronic devices process information and transduce energy with electrons, while biology performs such operations with ions and chemicals. To establish bio-device connectivity, we fabricate a redox-capacitor film from a polysaccharide (i.e., chitosan) and a redox-active catechol. We report that these films are rapidly and repeatedly charged and discharged electrochemically via a redox-cycling mechanism in which mediators shuttle electrons between the electrode and film (capacitance ˜ 40 F/g or 2.9 mF/cm2). Further, charging and discharging can be executed under bio-relevant conditions. Enzymatic-charging is achieved by electron-transfer from glucose to the film via an NADPH-mediated redox-cycling mechanism. Discharging occurs by electron-donation to O2 to generate H2O2 that serves as substrate for peroxidase-mediated biochemical reactions. Thus, these films offer the capability of inter-converting electrochemical and biochemical inputs/outputs. Among potential applications, we anticipate that catechol–chitosan redox-capacitor films could serve as circuit elements for molecular logic operations or for transducing bio-based chemical energy into electricity. (Adv. Funct. Mater. 2012, 22, 1409–1416. DOI: 10.1002/adfm.201101946).
Direct SERS detection of contaminants in a complex mixture: Rapid, single step screening for melamine in liquid infant formula
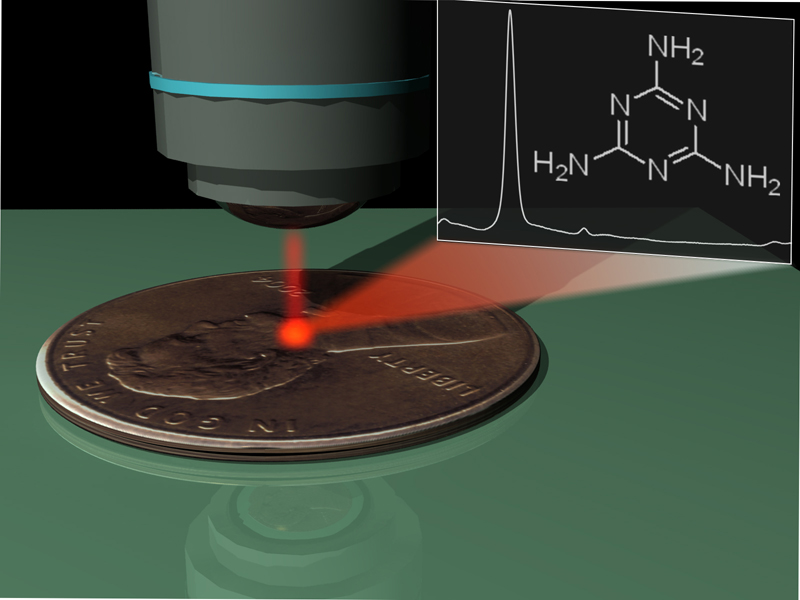 Melamine can be detected in infant formula without the need for additional sample preparation or purification using a simple galvanic displacement reaction to fabricate portable silver SERS substrates. The reaction is rapid, inexpensive, and robust enough to perform well on highly heterogeneous common metal objects such as tape and coins. (Analyst, 2012, 137, 826-828. DOI: 10.1039/C2AN15846A).
Melamine can be detected in infant formula without the need for additional sample preparation or purification using a simple galvanic displacement reaction to fabricate portable silver SERS substrates. The reaction is rapid, inexpensive, and robust enough to perform well on highly heterogeneous common metal objects such as tape and coins. (Analyst, 2012, 137, 826-828. DOI: 10.1039/C2AN15846A).
Coupling Electrodeposition with Layer-by-Layer Assembly to Address Proteins within Microfluidic Channels
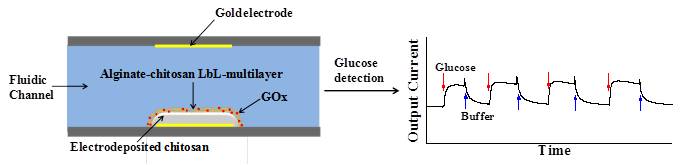 We couple two thin-film assembly methods to address proteins. Electrodeposition confers programmability and generates a template for layer-by-layer (LbL) assembly. LbL provides precise control of film thickness and the incorporation of labile biological components. The capabilities are demonstrated using glucose oxidase (GOx) based electrochemical biosensing within a microfabricated fluidic device. (Adv. Mater., 2011, 23, 5817-5823. DOI: 10.1002/adma.201103726).
We couple two thin-film assembly methods to address proteins. Electrodeposition confers programmability and generates a template for layer-by-layer (LbL) assembly. LbL provides precise control of film thickness and the incorporation of labile biological components. The capabilities are demonstrated using glucose oxidase (GOx) based electrochemical biosensing within a microfabricated fluidic device. (Adv. Mater., 2011, 23, 5817-5823. DOI: 10.1002/adma.201103726).
Electroaddressing Agarose Using Fmoc-Phenylalanine as Temporary Scaffold
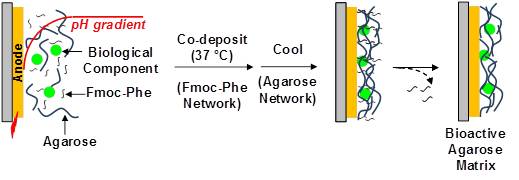 Electroaddressing, the use of imposed electrical stimuli to guide assembly, is attractive because electrical stimuli can be conveniently applied with high spatial and temporal resolution. Several electroaddressing mechanisms have been reported in which electrode-induced pH-gradients trigger stimuli-responsive materials to undergo localized sol-gel transitions to form hydrogel matrices. A common feature of existing hydrogel electrodeposition mechanisms is that the deposited matrix retains residual charged, acidic or basic (macro)molecules. Here, we report that the pH-responsive fluorenyl-9-methoxycarbonyl-phenylalanine (Fmoc-Phe) can be used to co-deposit the neutral and thermally-responsive polysaccharide agarose. Upon cooling, an agarose network is generated and the Fmoc-Phe can be removed. The Fmoc-Phe-mediated co-deposition of agarose is simple, rapid, spatially-selective, and allows for the electroaddressing of a bioactive matrix. (Langmuir, 2011, 27, 7380-7384. DOI: 10.1021/la201541c).
Electroaddressing, the use of imposed electrical stimuli to guide assembly, is attractive because electrical stimuli can be conveniently applied with high spatial and temporal resolution. Several electroaddressing mechanisms have been reported in which electrode-induced pH-gradients trigger stimuli-responsive materials to undergo localized sol-gel transitions to form hydrogel matrices. A common feature of existing hydrogel electrodeposition mechanisms is that the deposited matrix retains residual charged, acidic or basic (macro)molecules. Here, we report that the pH-responsive fluorenyl-9-methoxycarbonyl-phenylalanine (Fmoc-Phe) can be used to co-deposit the neutral and thermally-responsive polysaccharide agarose. Upon cooling, an agarose network is generated and the Fmoc-Phe can be removed. The Fmoc-Phe-mediated co-deposition of agarose is simple, rapid, spatially-selective, and allows for the electroaddressing of a bioactive matrix. (Langmuir, 2011, 27, 7380-7384. DOI: 10.1021/la201541c).
Reversible Electroaddressing of Self-Assembling Amino Acid Conjugate
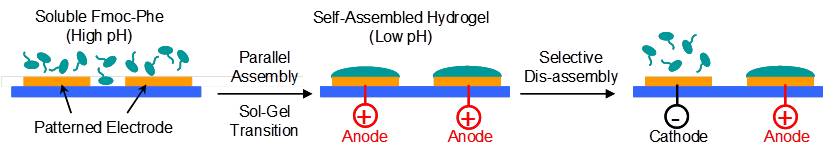 pH-responsive Fmoc-phenylalanine can be reversibly assembled (i.e., electrodeposited) in response to anodic signals and disassembled (i.e., dissolved) in response to cathodic signals. Fmoc-phenylalanine assembly/disassembly on patterned electrode addresses is rapid, spatially-controllable, programmable, and can be achieved in a covered microfluidic channel. Reversible electroaddressing should be especially useful for enlisting localized electrical signals in lab-on-a-chip applications. (Adv. Funct. Mater., 2011, 21, 1575-1580. DOI: 10.1002/adfm.201002020).
pH-responsive Fmoc-phenylalanine can be reversibly assembled (i.e., electrodeposited) in response to anodic signals and disassembled (i.e., dissolved) in response to cathodic signals. Fmoc-phenylalanine assembly/disassembly on patterned electrode addresses is rapid, spatially-controllable, programmable, and can be achieved in a covered microfluidic channel. Reversible electroaddressing should be especially useful for enlisting localized electrical signals in lab-on-a-chip applications. (Adv. Funct. Mater., 2011, 21, 1575-1580. DOI: 10.1002/adfm.201002020).
Redox-Cycling and H2O2-Generation by Fabricated Catecholic Films in the Absence of Enzymes
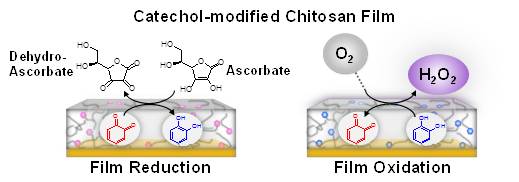 Phenolic matrices are ubiquitous in nature (e.g., lignin, melanin and humics) but their insolubility and structural complexity limits study of their activities and their functions. We fabricated an abiotic catechol-polysaccharide matrix in two steps; (i) electrodeposition of a film of the aminopolysaccharide chitosan onto a gold electrode, and (ii) electrochemical modification of the film with catechol. The catechol-modified chitosan films possess interesting redox-activities: (i) they can be repeatedly switched between their oxidized and reduced states; (ii) they can accept electrons from biological reductants (e.g., ascorbate and NADPH); (iii) they can donate electrons in a model biological oxidation process as well as in electrochemical redox cycling; and (iv) they can donate electrons to O2 to generate H2O2. The demonstration that abiotic catechol-chitosan films possess catalytic activities in the absence of enzymes suggests the possibility that phenolic matrices may play an important role in redox cycling and ROS-signaling in biology and the environment. (Biomacromolecules 2011, 12, 880-888, DOI: 10.1021/bm101499a)
Phenolic matrices are ubiquitous in nature (e.g., lignin, melanin and humics) but their insolubility and structural complexity limits study of their activities and their functions. We fabricated an abiotic catechol-polysaccharide matrix in two steps; (i) electrodeposition of a film of the aminopolysaccharide chitosan onto a gold electrode, and (ii) electrochemical modification of the film with catechol. The catechol-modified chitosan films possess interesting redox-activities: (i) they can be repeatedly switched between their oxidized and reduced states; (ii) they can accept electrons from biological reductants (e.g., ascorbate and NADPH); (iii) they can donate electrons in a model biological oxidation process as well as in electrochemical redox cycling; and (iv) they can donate electrons to O2 to generate H2O2. The demonstration that abiotic catechol-chitosan films possess catalytic activities in the absence of enzymes suggests the possibility that phenolic matrices may play an important role in redox cycling and ROS-signaling in biology and the environment. (Biomacromolecules 2011, 12, 880-888, DOI: 10.1021/bm101499a)
Biocompatible multi-address 3D cell assembly in microfluidic devices using spatially programmable gel formation
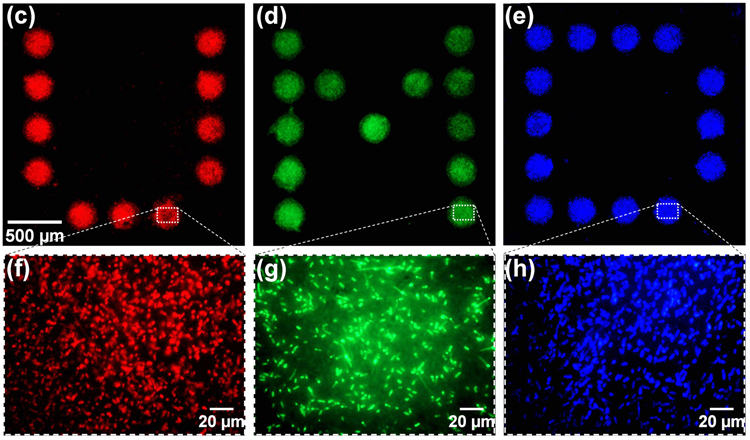 Programmable 3D cell assembly under physiological pH conditions is achieved using electrodeposited stimuli-responsive alginate gels in a microfluidic device, with parallel sidewall electrodes enabling direct observation of the cell assembly. Electrically triggered assembly and subsequent viability of mammalian cells is demonstrated, along with spatially programmable, multi-address assembly of different strains of E. coli cells. Our approach enables in vitro study of dynamic cellular and inter-cellular processes, from cell growth and stimulus/response to inter-colony and inter-species signaling. (Lab Chip, 2011, 11, 2316-2318. DOI: 10.1039/C1LC20306A).
Programmable 3D cell assembly under physiological pH conditions is achieved using electrodeposited stimuli-responsive alginate gels in a microfluidic device, with parallel sidewall electrodes enabling direct observation of the cell assembly. Electrically triggered assembly and subsequent viability of mammalian cells is demonstrated, along with spatially programmable, multi-address assembly of different strains of E. coli cells. Our approach enables in vitro study of dynamic cellular and inter-cellular processes, from cell growth and stimulus/response to inter-colony and inter-species signaling. (Lab Chip, 2011, 11, 2316-2318. DOI: 10.1039/C1LC20306A).
Mechanism of anodic electrodeposition of calcium alginate
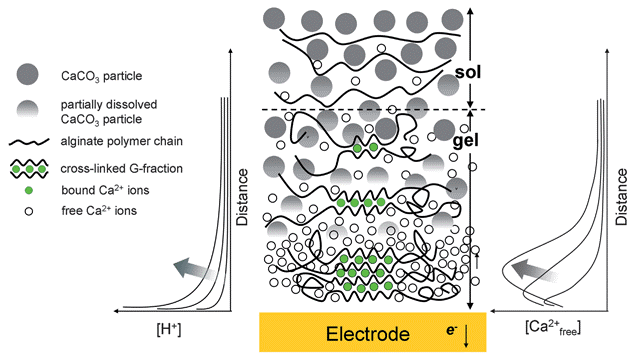 Stimuli-responsive polysaccharides that can undergo a sol-gel transition in response to localized electrical signals provide a unique opportunity to electroaddress biological components at device interfaces. Most polysaccharide electroaddressing mechanisms use electrochemical reactions to generate pH gradients that can locally neutralize the polysaccharide and induce its reversible sol-gel transition to form a hydrogel film adjacent to the electrode surface. The calcium-responsive polysaccharide alginate is an exception; it may electrodeposit without requiring extreme pH gradients and thus may provide a means to electroaddress pH-sensitive biological components. Here, we use a novel device to characterize the mechanism for the anodic electrodeposition of a calcium alginate hydrogel. This device consists of a transparent fluidic channel with built-in sidewall electrodes that allows Ca-alginate electrodeposition to be directly measured by non-destructive optical and spectroscopic methods. We verified the electrodeposition mechanism using; pH-responsive dyes to observe the local pH gradients during gel formation, Ca2+ indicator dyes to observe the Ca2+ gradient; and in situ Raman spectroscopy to demonstrate a strong interaction between soluble Ca2+ and alginate. Importantly, these results demonstrate electrodeposition without the need for a substantial pH excursion from neutrality. Thus, calcium alginate appears especially well-suited for electroaddressing labile biological components for applications in biosensors, biofabrication and bioMEMS. (Soft Matter, 2011, 7, 5677-5684. DOI: 10.1039/c1sm05210a).
Stimuli-responsive polysaccharides that can undergo a sol-gel transition in response to localized electrical signals provide a unique opportunity to electroaddress biological components at device interfaces. Most polysaccharide electroaddressing mechanisms use electrochemical reactions to generate pH gradients that can locally neutralize the polysaccharide and induce its reversible sol-gel transition to form a hydrogel film adjacent to the electrode surface. The calcium-responsive polysaccharide alginate is an exception; it may electrodeposit without requiring extreme pH gradients and thus may provide a means to electroaddress pH-sensitive biological components. Here, we use a novel device to characterize the mechanism for the anodic electrodeposition of a calcium alginate hydrogel. This device consists of a transparent fluidic channel with built-in sidewall electrodes that allows Ca-alginate electrodeposition to be directly measured by non-destructive optical and spectroscopic methods. We verified the electrodeposition mechanism using; pH-responsive dyes to observe the local pH gradients during gel formation, Ca2+ indicator dyes to observe the Ca2+ gradient; and in situ Raman spectroscopy to demonstrate a strong interaction between soluble Ca2+ and alginate. Importantly, these results demonstrate electrodeposition without the need for a substantial pH excursion from neutrality. Thus, calcium alginate appears especially well-suited for electroaddressing labile biological components for applications in biosensors, biofabrication and bioMEMS. (Soft Matter, 2011, 7, 5677-5684. DOI: 10.1039/c1sm05210a).
In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes
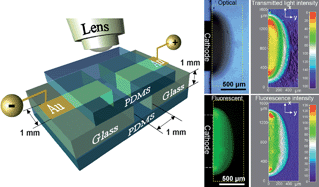 We report the first in situ quantitative visualization and characterization of electro-induced chitosan hydrogel growth in an aqueous environment. This was enabled with a pair of sidewall electrodes within a transparent fluidic system, which allowed us to resolve the electrogelling mechanism and interpret the dominant causes responsible for the formation and density distribution of the deposited hydrogel. The pH and the time-dependent growth profiles of the chitosan hydrogel were directly visualized, analyzed, and characterized. The results indicate that the gelation and immobilization of chitosan onto the cathode at a pH above its pKa value (~6.3) are due to the electrochemically generated concentration gradient of reactant OH- ions, and their subsequent neutralization of the NH3+ groups of chitosan chains in solution near the cathode. The increased gel density around the fringes of the electrodes was demonstrated and correlated with the electrophoretic migration of chitosan cations during deposition. Simulation of the electric potential/field distribution, together with the corresponding dry film topography confirmed the non-uniform, electric field-dependent density distribution of deposited hydrogel. This report provides fundamental understanding towards the mechanism and the kinetics of the electro-induced chitosan gel formation. It also provides important guidelines for pursuing its application in bio-components integrated microsystems. The method in use exemplifies a simple, effective and non-destructive approach for in situ characterization of electro-responsive biopolymers in an aqueous environment. (Soft Matter, 2010, 6, 3177-3183; DOI: 10.1039/c0sm00124d).
We report the first in situ quantitative visualization and characterization of electro-induced chitosan hydrogel growth in an aqueous environment. This was enabled with a pair of sidewall electrodes within a transparent fluidic system, which allowed us to resolve the electrogelling mechanism and interpret the dominant causes responsible for the formation and density distribution of the deposited hydrogel. The pH and the time-dependent growth profiles of the chitosan hydrogel were directly visualized, analyzed, and characterized. The results indicate that the gelation and immobilization of chitosan onto the cathode at a pH above its pKa value (~6.3) are due to the electrochemically generated concentration gradient of reactant OH- ions, and their subsequent neutralization of the NH3+ groups of chitosan chains in solution near the cathode. The increased gel density around the fringes of the electrodes was demonstrated and correlated with the electrophoretic migration of chitosan cations during deposition. Simulation of the electric potential/field distribution, together with the corresponding dry film topography confirmed the non-uniform, electric field-dependent density distribution of deposited hydrogel. This report provides fundamental understanding towards the mechanism and the kinetics of the electro-induced chitosan gel formation. It also provides important guidelines for pursuing its application in bio-components integrated microsystems. The method in use exemplifies a simple, effective and non-destructive approach for in situ characterization of electro-responsive biopolymers in an aqueous environment. (Soft Matter, 2010, 6, 3177-3183; DOI: 10.1039/c0sm00124d).
Engineered biological nanofactories trigger quorum sensing response in targeted bacteria
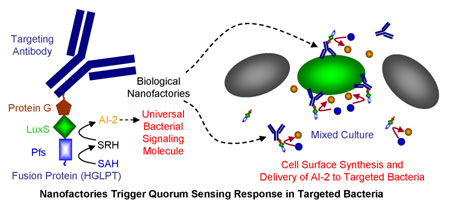 The creation of self-assembled biological nanofactories made up of targeting, sensing, synthesis and assembly modules for selectively triggering the quorum sensing response of targeted bacteria is demonstrated. Targeting is achieved by a bacteria-specific targeting antibody. Sensing, synthesis and assembly is achieved by a fusion construct (His)6–Protein G–LuxS–Pfs-(Tyr)5, called HGLPT. Protein G facilitates attachment of HGLPT to the targeting antibody while Pfs and LuxS synthesize AI-2. When deployed, the nanofactories bind to targeted bacteria in mixed cultures, synthesize AI-2 there and elicit their quorum sensing response. Prospects for creation of next generational anti-microbials based on quorum sensing modulation are envisioned (Nature Nanotechnol., doi 10.1038/nnano.2009.457)
The creation of self-assembled biological nanofactories made up of targeting, sensing, synthesis and assembly modules for selectively triggering the quorum sensing response of targeted bacteria is demonstrated. Targeting is achieved by a bacteria-specific targeting antibody. Sensing, synthesis and assembly is achieved by a fusion construct (His)6–Protein G–LuxS–Pfs-(Tyr)5, called HGLPT. Protein G facilitates attachment of HGLPT to the targeting antibody while Pfs and LuxS synthesize AI-2. When deployed, the nanofactories bind to targeted bacteria in mixed cultures, synthesize AI-2 there and elicit their quorum sensing response. Prospects for creation of next generational anti-microbials based on quorum sensing modulation are envisioned (Nature Nanotechnol., doi 10.1038/nnano.2009.457)
Cross Species Quorum Quenching Using A Native AI-2 Processing Enzyme
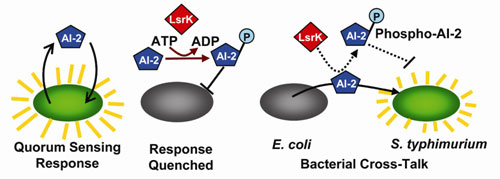 We present a technique for interrupting communication among bacteria that exploits
their native and highly specific machinery for processing the signaling molecules themselves. Specifically, our approach is to bring native intracellular signal processing mechanisms to the extracellular surroundings and “quench” crosstalk among a variety of strains. We demonstrate that the Escherichia coli AI-2 kinase, LsrK, can phosphorylate AI-2 in vitro, and when LsrK treated AI-2 is added ex vivo to E. coli populations, the native QS response is significantly reduced. Analogous results are obtained from a synthetic ecosystem where three species of bacteria (enteric and marine) are co-cultured. Finally, the addition of LsrK and ATP to growing co-cultures of E. coli and S. typhimurium, exhibits significantly reduced native “cross-talk” that ordinarily exists among and between species in an ecosystem. We believe this nature-inspired enzymatic approach for quenching QS systems will spawn new methods for controlling cell phenotype and potentially open new avenues for controlling bacterial pathogenicity. (ACS Chem Biol (2010), DOI: 10.1021 /cb9002738)
We present a technique for interrupting communication among bacteria that exploits
their native and highly specific machinery for processing the signaling molecules themselves. Specifically, our approach is to bring native intracellular signal processing mechanisms to the extracellular surroundings and “quench” crosstalk among a variety of strains. We demonstrate that the Escherichia coli AI-2 kinase, LsrK, can phosphorylate AI-2 in vitro, and when LsrK treated AI-2 is added ex vivo to E. coli populations, the native QS response is significantly reduced. Analogous results are obtained from a synthetic ecosystem where three species of bacteria (enteric and marine) are co-cultured. Finally, the addition of LsrK and ATP to growing co-cultures of E. coli and S. typhimurium, exhibits significantly reduced native “cross-talk” that ordinarily exists among and between species in an ecosystem. We believe this nature-inspired enzymatic approach for quenching QS systems will spawn new methods for controlling cell phenotype and potentially open new avenues for controlling bacterial pathogenicity. (ACS Chem Biol (2010), DOI: 10.1021 /cb9002738)
Magnetic nanofactories: Localized synthesis and delivery of quorum-sensing signaling molecule autoinducer-2 to bacterial cell surfaces
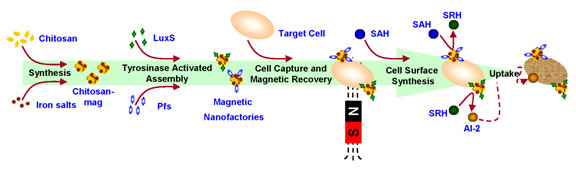 Magnetic nanofactories that are comprised of chitosan-mag nanoparticles and AI-2 synthesis enzymes, Pfs and LuxS, are demonstrated to alter the response of captured bacteria via localized synthesis and delivery of the signal molecule AI-2. The biopolymer chitosan (in the chitosan-mag nanoparticles) facilitates capture of the bacteria and serves as a platform for the attachment of the enzymes Pfs and LuxS via their genetically engineered C-terminal tyrosine tags. Pfs and LuxS synthesize AI-2 at the surface of the captured bacteria and the magnetic particles facilitate recovery of bound bacteria (Metab. Eng., 2007, 9, 228-239).
Magnetic nanofactories that are comprised of chitosan-mag nanoparticles and AI-2 synthesis enzymes, Pfs and LuxS, are demonstrated to alter the response of captured bacteria via localized synthesis and delivery of the signal molecule AI-2. The biopolymer chitosan (in the chitosan-mag nanoparticles) facilitates capture of the bacteria and serves as a platform for the attachment of the enzymes Pfs and LuxS via their genetically engineered C-terminal tyrosine tags. Pfs and LuxS synthesize AI-2 at the surface of the captured bacteria and the magnetic particles facilitate recovery of bound bacteria (Metab. Eng., 2007, 9, 228-239).
AI-2 biosynthesis module in a magnetic nanofactory alters bacterial response via localized synthesis and delivery
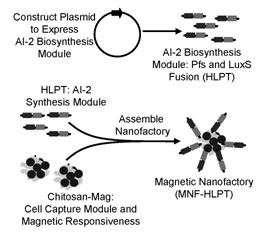 Fusion protein (His)6-LuxS-Pfs-(Tyr)5, abbreviated as HLPT, is constructed to express the AI-2 synthesis enzymes Pfs and LuxS in one fusion protein. HLPT is demonstrated to be more active than equimolar amounts of the constituent enzymes over a wide range of experimental conditions (synthesis temperatures and reaction times). The resultant HLPT based nanofactory is demonstrated to alter the native response of captured bacteria (Biotechnol. Bioeng., 109, 2, 390-399).
Fusion protein (His)6-LuxS-Pfs-(Tyr)5, abbreviated as HLPT, is constructed to express the AI-2 synthesis enzymes Pfs and LuxS in one fusion protein. HLPT is demonstrated to be more active than equimolar amounts of the constituent enzymes over a wide range of experimental conditions (synthesis temperatures and reaction times). The resultant HLPT based nanofactory is demonstrated to alter the native response of captured bacteria (Biotechnol. Bioeng., 109, 2, 390-399).
Biofabrication of antibodies and antigens via IgG-binding domain engineered with activatable pentatyrosine pro-tag
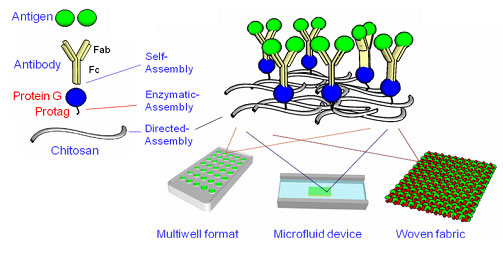 The capability biologically-based fabrication (i.e., biofabrication) provides bottom-up hierarchical assembly of functional supramolecular structures that could be designed to facilitate integration into devices. Specifically, we create an engineered IgG-binding domain (HG3T) with a C-terminal enzyme-activatable pentatyrosine “pro-tag” that allows covalent coupling to the pH stimuli-responsive polysaccharide, chitosan, which is spatially addressed through electro-signal. Different configurations of formats, such as multiwell plates, micropatterned electrodes, and fiber networks are demonstrated and loading of both HG3T and antibodies can be achieved in a linear fashion so that quantitative assessment of antibodies and antigens is feasible. (Biotechnology and Bioengineering, 2009, 103, 231-40)
The capability biologically-based fabrication (i.e., biofabrication) provides bottom-up hierarchical assembly of functional supramolecular structures that could be designed to facilitate integration into devices. Specifically, we create an engineered IgG-binding domain (HG3T) with a C-terminal enzyme-activatable pentatyrosine “pro-tag” that allows covalent coupling to the pH stimuli-responsive polysaccharide, chitosan, which is spatially addressed through electro-signal. Different configurations of formats, such as multiwell plates, micropatterned electrodes, and fiber networks are demonstrated and loading of both HG3T and antibodies can be achieved in a linear fashion so that quantitative assessment of antibodies and antigens is feasible. (Biotechnology and Bioengineering, 2009, 103, 231-40)
In situ generation of pH gradients in microfluidic devices for biofabrication of freestanding, semi-permeable chitosan membranes
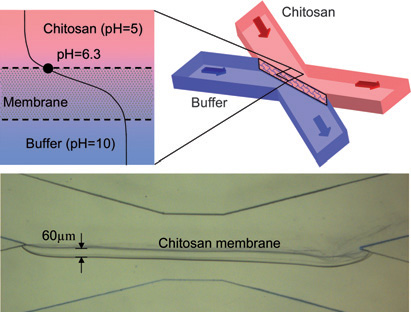 The pH-responsive polysaccharide chitosan was used to form a freestanding membrane structure in microfluidic networks. pH gradients are generated at the converging interface between a slightly acidic chitosan solution and a slightly basic buffer solution. The chitosan membranes were about 30–60 mm thick and uniform inside the microchannels. The membranes are permeable to aqueous solutions and are removed by mildly acidic solutions. Permeability tests suggested that the membrane pore size was a few nanometres, i.e., the size range of antibodies. We believe that the facile, rapid biofabrication of freestanding chitosan membranes can be applied to many biochemical, bioanalytical, biosensing applications and cellular studies. (Lab Chip, 2010, 10, 1, 59-65.)
The pH-responsive polysaccharide chitosan was used to form a freestanding membrane structure in microfluidic networks. pH gradients are generated at the converging interface between a slightly acidic chitosan solution and a slightly basic buffer solution. The chitosan membranes were about 30–60 mm thick and uniform inside the microchannels. The membranes are permeable to aqueous solutions and are removed by mildly acidic solutions. Permeability tests suggested that the membrane pore size was a few nanometres, i.e., the size range of antibodies. We believe that the facile, rapid biofabrication of freestanding chitosan membranes can be applied to many biochemical, bioanalytical, biosensing applications and cellular studies. (Lab Chip, 2010, 10, 1, 59-65.)
Spatial resolution in chitosan-based programmable biomolecular scaffolds
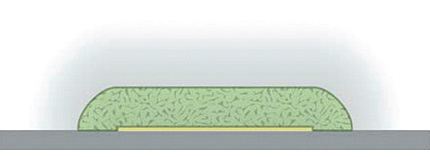 The polysaccharide chitosan is a versatile scaffold for assembly and conjugation of biomolecules, including nucleic acids, proteins, and viruses. It provides unique value in bioMEMS in that it can be electrodeposited on demand at specific locations in closed microfluidic systems, enabling spatial and temporal programmability of biomolecular binding and reaction sites in bio-microfluidic networks. In anticipating the scaling of such systems to smaller dimensions, we have investigated the spatial resolution which the chitosan electrodeposition process can achieve, using micron-scale electrode patterns coupled with fluorescence and Raman microscopies to assess the fidelity and edge sharpness of the resulting chitosan patterns. These show that electrodeposited chitosan scaffolds can be defined to an edge sharpness of 0.5–1.0 mm, compatible with the vast majority of microfluidics and bioMEMS applications, where microfluidic channel dimensions in the range of 10s or 100s of micrometers are common. (Soft Matter, 2009, 5, 3677-3681.)
The polysaccharide chitosan is a versatile scaffold for assembly and conjugation of biomolecules, including nucleic acids, proteins, and viruses. It provides unique value in bioMEMS in that it can be electrodeposited on demand at specific locations in closed microfluidic systems, enabling spatial and temporal programmability of biomolecular binding and reaction sites in bio-microfluidic networks. In anticipating the scaling of such systems to smaller dimensions, we have investigated the spatial resolution which the chitosan electrodeposition process can achieve, using micron-scale electrode patterns coupled with fluorescence and Raman microscopies to assess the fidelity and edge sharpness of the resulting chitosan patterns. These show that electrodeposited chitosan scaffolds can be defined to an edge sharpness of 0.5–1.0 mm, compatible with the vast majority of microfluidics and bioMEMS applications, where microfluidic channel dimensions in the range of 10s or 100s of micrometers are common. (Soft Matter, 2009, 5, 3677-3681.)
Design optimization for bioMEMS studies of enzyme-controlled metabolic pathways
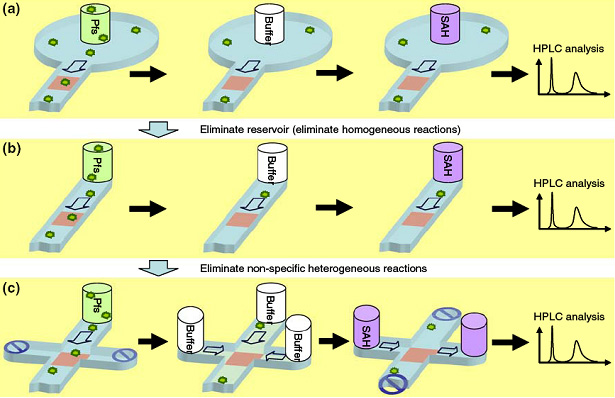 We have previously shown that electrodeposited chitosan enables immobilization of an enzyme at a specific site while maintaining its catalytic activity. These investigations also revealed parasitic effects, including products generated by the enzyme either in the liquid or nonspecifically bound to microchannel surfaces. To reduce reactions in the liquid, we have developed new packaging which eliminates fluid reservoirs that are commonly used. To reduce reactions from enzymes on microchannel walls, we used a cross-flow design so that flows for enzyme assembly and enzymatic reaction share only the intended reaction site. Our results show that the signal-to-background ratio of sequential enzymatic reactions increases by eliminating the reservoirs and further increases by separating the flow direction of enzymatic reaction from that of enzyme assembly step. These techniques can be easily applied to versatile microfluidic devices to minimize parasitic reactions in sequential biochemical reactions. (Biomed. Microdev., 2008, 10, 899-908.)
We have previously shown that electrodeposited chitosan enables immobilization of an enzyme at a specific site while maintaining its catalytic activity. These investigations also revealed parasitic effects, including products generated by the enzyme either in the liquid or nonspecifically bound to microchannel surfaces. To reduce reactions in the liquid, we have developed new packaging which eliminates fluid reservoirs that are commonly used. To reduce reactions from enzymes on microchannel walls, we used a cross-flow design so that flows for enzyme assembly and enzymatic reaction share only the intended reaction site. Our results show that the signal-to-background ratio of sequential enzymatic reactions increases by eliminating the reservoirs and further increases by separating the flow direction of enzymatic reaction from that of enzyme assembly step. These techniques can be easily applied to versatile microfluidic devices to minimize parasitic reactions in sequential biochemical reactions. (Biomed. Microdev., 2008, 10, 899-908.)
Programmable assembly of a metabolic pathway enzyme in a pre-packaged reusable bioMEMS device
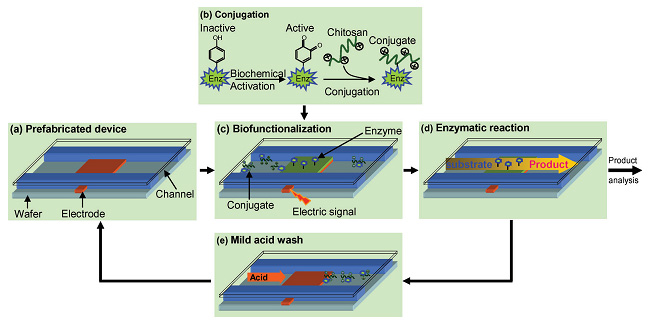 We report a strategy for the assembly of active enzymes within a completely packaged bioMEMS device through the generation of electrical signals at spatially and temporally defined sites. An enzyme from a bacterial metabolic pathway is genetically fused with a “pro-tag” that allows it to be covalently bound to the biopolymer chitosan. Assembly is based on electrodeposition of the enzyme–chitosan conjugate onto addressable sites in microfluidic channels. Compared to traditional enzyme immobilization approaches in microfluidics, our approach assembles enzymes under mild aqueous conditions with spatial and temporal programmability and orientational control. The assembly can be reversed, making the bioMEMS reusable. The assembled enzymes retain catalytic activity over multiple days, demonstrating enhanced stability. We envision that this assembly strategy can be applied to rebuild metabolic pathways in microfluidic environments for antimicrobial drug discovery. (Lab Chip, 2008, 8, 420-430.)
We report a strategy for the assembly of active enzymes within a completely packaged bioMEMS device through the generation of electrical signals at spatially and temporally defined sites. An enzyme from a bacterial metabolic pathway is genetically fused with a “pro-tag” that allows it to be covalently bound to the biopolymer chitosan. Assembly is based on electrodeposition of the enzyme–chitosan conjugate onto addressable sites in microfluidic channels. Compared to traditional enzyme immobilization approaches in microfluidics, our approach assembles enzymes under mild aqueous conditions with spatial and temporal programmability and orientational control. The assembly can be reversed, making the bioMEMS reusable. The assembled enzymes retain catalytic activity over multiple days, demonstrating enhanced stability. We envision that this assembly strategy can be applied to rebuild metabolic pathways in microfluidic environments for antimicrobial drug discovery. (Lab Chip, 2008, 8, 420-430.)
A Cantilever Sensor with an Integrated Optical Readout for Detection of Enzymatically Produced Homocysteine
 Microcantilever sensors have been fabricated within microfluidic channels to detect the binding of enzymatically produced Homocysteine, a byproduct of bacterial AI-2 production. The cantilevers are patterned in 2.2 µm thick SU-8 and also act as waveguides for coupling red laser light at 633 nm through the device. The cantilevers are coated with a thin gold layer which acts as a binding surface for the thiol-containing compound, Homocysteine. The bending of the cantilever is determined by the change in the coupled optical power, eliminating the need for off-chip free space optics. Cantilever displacement as low as 5 nm correlating to a surface stress of 1 mN/m could be detected with the device. (IEEE TBCAS, 2009, 3, 415)
Microcantilever sensors have been fabricated within microfluidic channels to detect the binding of enzymatically produced Homocysteine, a byproduct of bacterial AI-2 production. The cantilevers are patterned in 2.2 µm thick SU-8 and also act as waveguides for coupling red laser light at 633 nm through the device. The cantilevers are coated with a thin gold layer which acts as a binding surface for the thiol-containing compound, Homocysteine. The bending of the cantilever is determined by the change in the coupled optical power, eliminating the need for off-chip free space optics. Cantilever displacement as low as 5 nm correlating to a surface stress of 1 mN/m could be detected with the device. (IEEE TBCAS, 2009, 3, 415)
An Optical MEMS Sensor Utilizing a Chitosan Film for Catechol Detection
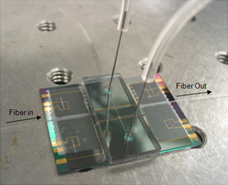 A microfluidic device for the detection of the phenol catechol has been fabricated and tested. Polymer waveguides are patterned using SU-8 to guide blue laser light through the encapsulated liquid channel. The light passes through an electrodeposited chitosan film. Catechol is electrochemically oxidized in the channel and its byproducts bind to the chitosan film inducing an absorbance change. The measured light intensity at the output can be correlated to the concentration of catechol in the channel. (Sens. Actuators, B, 2009, B138, 64)
A microfluidic device for the detection of the phenol catechol has been fabricated and tested. Polymer waveguides are patterned using SU-8 to guide blue laser light through the encapsulated liquid channel. The light passes through an electrodeposited chitosan film. Catechol is electrochemically oxidized in the channel and its byproducts bind to the chitosan film inducing an absorbance change. The measured light intensity at the output can be correlated to the concentration of catechol in the channel. (Sens. Actuators, B, 2009, B138, 64)
Mechano-transduction of DNA Hybridization and Dopamine Oxidation through Electrodeposited Chitosan Network
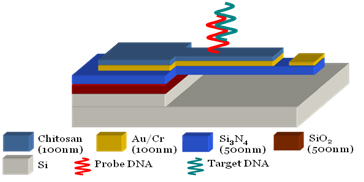 Microcantilevers only 100 µm long and 600 nm thick are fabricated to act as sensors of both DNA hybridization and dopamine detection. A film of chitosan is electrodeposited onto the cantilever beam to act as a functionalization layer. Binding of either target DNA to immobilized probe DNA or of oxidized dopamine to chitosan amine groups causes bending of the cantilever beam which is measured using an optical probe. (Lab on a Chip, 2008, 7, 103)
Microcantilevers only 100 µm long and 600 nm thick are fabricated to act as sensors of both DNA hybridization and dopamine detection. A film of chitosan is electrodeposited onto the cantilever beam to act as a functionalization layer. Binding of either target DNA to immobilized probe DNA or of oxidized dopamine to chitosan amine groups causes bending of the cantilever beam which is measured using an optical probe. (Lab on a Chip, 2008, 7, 103)
Electroaddressing of Cell Populations by Co-Deposition with Calcium Alginate Hydrogels
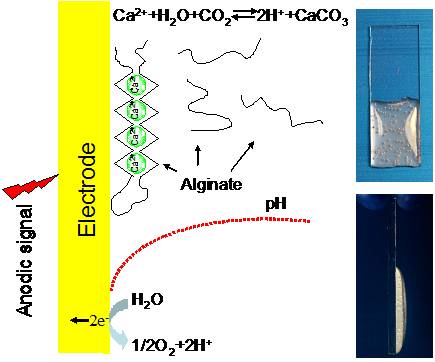 Calcium alginate hydrogels can be electrodeposited at electrode addresses without the need for reagents. Mechanistically, anodic signals trigger a localized solubilization of CaCO3 and the release of Ca2+ induces the spatially controlled formation of calcium alginate hydrogels. Alginate electrodeposition is sufficiently mild that bacterial cell populations co-deposited within the hydrogel are viable, grow, and respond to externally added inducers and signaling molecules. (Adv. Funct. Mater. 2009, 19, 2074.)
Calcium alginate hydrogels can be electrodeposited at electrode addresses without the need for reagents. Mechanistically, anodic signals trigger a localized solubilization of CaCO3 and the release of Ca2+ induces the spatially controlled formation of calcium alginate hydrogels. Alginate electrodeposition is sufficiently mild that bacterial cell populations co-deposited within the hydrogel are viable, grow, and respond to externally added inducers and signaling molecules. (Adv. Funct. Mater. 2009, 19, 2074.)
Biomimetic Sealant Based On Gelatin and Microbial Transglutaminase: An Initial In Vivo Investigation
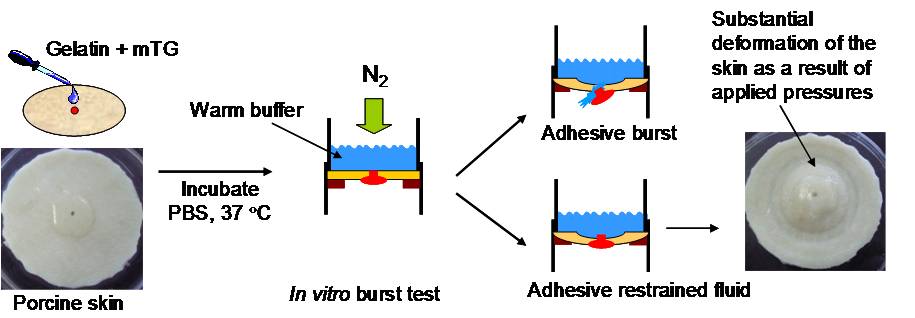 The potential of an in situ gel-forming adhesive was examined as a hemostatic surgical sealant. Specifically, gelatin is used as the structural protein and microbial transglutaminase (mTG) is used as the crosslinking enzyme. In vitro burst pressure tests with porcine skin demonstrate that the gelatin-mTG adhesive forms a gel under moist conditions and this gel can restrain pressures of 200 mmHg. In vivo tests showed the gelatin-mTG adhesive achieves complete hemostasis within 4 minutes and the gel offers substantial adhesive and cohesive strength. (J. Biomed. Mater. Res. B Appl. Biomater., 2009, 91B, 5.)
The potential of an in situ gel-forming adhesive was examined as a hemostatic surgical sealant. Specifically, gelatin is used as the structural protein and microbial transglutaminase (mTG) is used as the crosslinking enzyme. In vitro burst pressure tests with porcine skin demonstrate that the gelatin-mTG adhesive forms a gel under moist conditions and this gel can restrain pressures of 200 mmHg. In vivo tests showed the gelatin-mTG adhesive achieves complete hemostasis within 4 minutes and the gel offers substantial adhesive and cohesive strength. (J. Biomed. Mater. Res. B Appl. Biomater., 2009, 91B, 5.)
Chitosan-Coated Wires: Conferring Electrical Properties to Chitosan Fibers
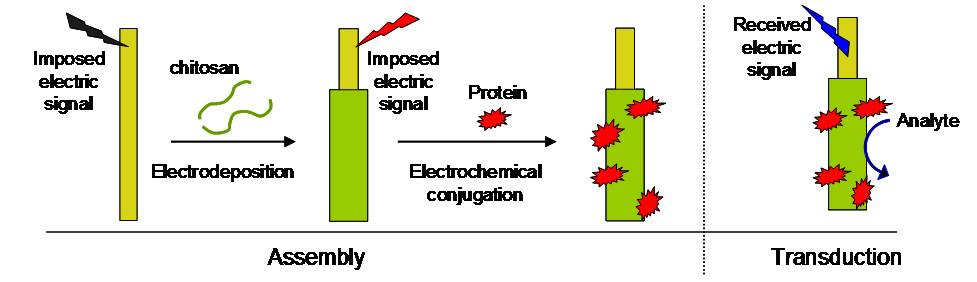 The capabilities of a polymeric fiber for facile biofunctionalization is coupled with the signal transduction capabilities of a conducting wire to generate a hybrid platform that can be viewed as either a biofunctionalized wire or a conducting fiber. Integral to this hybrid platform is the interface material chitosan that enables simple electrical signals to be employed to biofunctionalize conducting wires. Specifically, we use cathodic signals to direct chitosan to electrodeposit onto gold wires and anodic signals to conjugate proteins (e.g., for biosensing) to the chitosan-coated wire. In addition, the chitosan-coating is permeable to small molecules, which allows for the electrical detection of electrochemically active compounds that are either present in the external environment or generated by a biofunctionalized chitosan coating. The capabilities for biofunctionalization and transduction are demonstrated for the detection of glucose by chitosan-coated wires functionalized with the enzyme glucose oxidase. (Biomacromolecules, 2009, 10, 858.)
The capabilities of a polymeric fiber for facile biofunctionalization is coupled with the signal transduction capabilities of a conducting wire to generate a hybrid platform that can be viewed as either a biofunctionalized wire or a conducting fiber. Integral to this hybrid platform is the interface material chitosan that enables simple electrical signals to be employed to biofunctionalize conducting wires. Specifically, we use cathodic signals to direct chitosan to electrodeposit onto gold wires and anodic signals to conjugate proteins (e.g., for biosensing) to the chitosan-coated wire. In addition, the chitosan-coating is permeable to small molecules, which allows for the electrical detection of electrochemically active compounds that are either present in the external environment or generated by a biofunctionalized chitosan coating. The capabilities for biofunctionalization and transduction are demonstrated for the detection of glucose by chitosan-coated wires functionalized with the enzyme glucose oxidase. (Biomacromolecules, 2009, 10, 858.)
Orthogonal Enzymatic Reactions for the Assembly of Proteins at Electrode Addresses
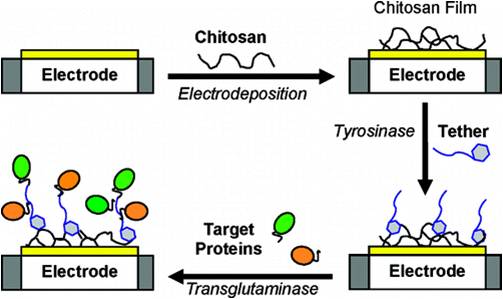 We enlist the unique capabilities of enzymes and biologically derived polymers to assemble target proteins to electrode addresses. First, the stimuli-responsive chitosan is directed to assemble at the electrode address in response to electrode-imposed signals. Next, tyrosinase is used to catalyze grafting of a protein or peptide tether to the chitosan film. Finally, microbial transglutaminase (mTG) catalyzes the assembly of target proteins to the tether. This assembly method employs the electrical signal to confer spatial selectivity (during chitosan electrodeposition) and employs the enzymes to confer chemical selectivity (i.e., amino acid residue selectivity). Further, this method is mild, since no reactive reagents or protection steps are required, and all steps are performed in aqueous solution. (Langmuir 2009, 25, 338.)
We enlist the unique capabilities of enzymes and biologically derived polymers to assemble target proteins to electrode addresses. First, the stimuli-responsive chitosan is directed to assemble at the electrode address in response to electrode-imposed signals. Next, tyrosinase is used to catalyze grafting of a protein or peptide tether to the chitosan film. Finally, microbial transglutaminase (mTG) catalyzes the assembly of target proteins to the tether. This assembly method employs the electrical signal to confer spatial selectivity (during chitosan electrodeposition) and employs the enzymes to confer chemical selectivity (i.e., amino acid residue selectivity). Further, this method is mild, since no reactive reagents or protection steps are required, and all steps are performed in aqueous solution. (Langmuir 2009, 25, 338.)
Reagentless Protein Assembly Triggered by Localized Electrical Signals
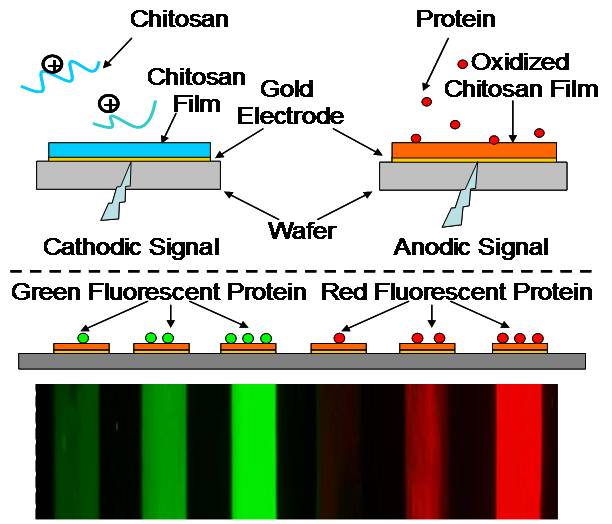 Electrode-imposed signals are used to assemble proteins without the need for reactive reagents. The two-step assembly approach uses i) cathodic signals to electrodeposit the chitosan and ii) anodic signals to activate the chitosan film for protein assembly. Proteins are shown to assemble at individual electrode addresses, with spatial selectivity and quantitative control. (Adv. Mater. 2009, 21, 984.)
Electrode-imposed signals are used to assemble proteins without the need for reactive reagents. The two-step assembly approach uses i) cathodic signals to electrodeposit the chitosan and ii) anodic signals to activate the chitosan film for protein assembly. Proteins are shown to assemble at individual electrode addresses, with spatial selectivity and quantitative control. (Adv. Mater. 2009, 21, 984.)
Chitosan-Coated Electrodes for Bimodal Sensing: Selective Post-Electrode Film Reaction for Spectroelectrochemical Analysis
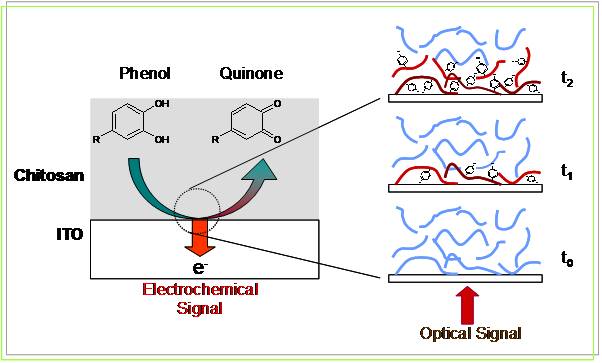 We report a novel approach that enables electrochemically initiated reactions to generate optical signals that can be used to enhance the discriminating power for the electrochemical analysis of antioxidant food phenols. This spectroelectro-chemical approach employs transparent electrodes coated with a film of chitosan. The phenolic analytes diffuse through the chitosan film to the electrode where they are anodically oxidized into electrophilic intermediates that undergo postelectrode reactions with the chitosan film. We demonstrate that the optical signal generated from the post-electrode film reaction is selective for oxidized phenols. Furthermore, we demonstrate that the optical signal can be correlated to the electrical signal. Finally, we use simple mixtures to demonstrate that the coupling of information from independent optical and electrical measurement modes can assist in the qualitative analysis of antioxidant phenols. (Langmuir, 2008, 24, 7223.)
We report a novel approach that enables electrochemically initiated reactions to generate optical signals that can be used to enhance the discriminating power for the electrochemical analysis of antioxidant food phenols. This spectroelectro-chemical approach employs transparent electrodes coated with a film of chitosan. The phenolic analytes diffuse through the chitosan film to the electrode where they are anodically oxidized into electrophilic intermediates that undergo postelectrode reactions with the chitosan film. We demonstrate that the optical signal generated from the post-electrode film reaction is selective for oxidized phenols. Furthermore, we demonstrate that the optical signal can be correlated to the electrical signal. Finally, we use simple mixtures to demonstrate that the coupling of information from independent optical and electrical measurement modes can assist in the qualitative analysis of antioxidant phenols. (Langmuir, 2008, 24, 7223.)
Chitosan Fibers: Versatile Platform for Nickel-Mediated Protein Assembly
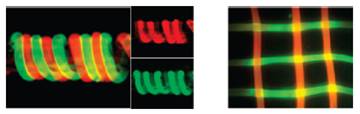 We report a method to biofunctionalize individual fibers by the reversible binding of proteins, and we suggest the potential of fiber assemblies by generating simple multifiber structures. Specifically, we use chitosan fibers and show that nickel can mediate assembly of histidine-tagged proteins to these fibers. Initial studies with the model His-GFP demonstrate the concept of nickel-mediated protein assembly. Subsequent studies with a His-tagged streptococcal antibody-binding protein (protein G) demonstrate the assembly of antibodies to generate antibody-presenting fibers. Antibody assembly onto the fiber was shown to be controllable. Importantly, antibody and antigen were observed to penetrate substantially into the individual fibers (tens of microns) to allow the assembly of pmole levels of protein per cm of fiber length. Finally, antibody-presenting fibers with different specificities were assembled into simple one- and two-dimensional structures, and individual fibers in these fiber assemblies were observed to capture their respective antigens from antigen mixtures. (Biomacromolecules 2008, 9, 1417.)
We report a method to biofunctionalize individual fibers by the reversible binding of proteins, and we suggest the potential of fiber assemblies by generating simple multifiber structures. Specifically, we use chitosan fibers and show that nickel can mediate assembly of histidine-tagged proteins to these fibers. Initial studies with the model His-GFP demonstrate the concept of nickel-mediated protein assembly. Subsequent studies with a His-tagged streptococcal antibody-binding protein (protein G) demonstrate the assembly of antibodies to generate antibody-presenting fibers. Antibody assembly onto the fiber was shown to be controllable. Importantly, antibody and antigen were observed to penetrate substantially into the individual fibers (tens of microns) to allow the assembly of pmole levels of protein per cm of fiber length. Finally, antibody-presenting fibers with different specificities were assembled into simple one- and two-dimensional structures, and individual fibers in these fiber assemblies were observed to capture their respective antigens from antigen mixtures. (Biomacromolecules 2008, 9, 1417.)
Chitosan Biotinylation and Electrodeposition for Selective Protein Assembly
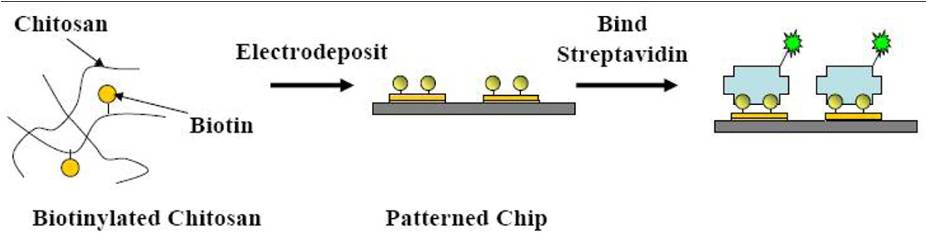 An alternative route to protein assembly at surfaces based on using the unique capabilities of biological materials for the spatially selective assembly of proteins is described. Specifically, the stimuli-responsive properties of amino-polysaccharide chitosan are combined with the molecular-recognition capabilities of biotin-streptavidin binding. Biotinylated chitosan retains its stimuli-responsive properties and is capable of electrodepositing at specific electrode addresses. Once deposited, it is capable of binding streptavidin, which can mediate the subsequent assembly of biotinylated proteins. Spatially selective protein assembly using biotinylated Protein A and fluorescently-labeled antibodies is demonstrated. (Macromol. Biosci. 2008, 8, 451.)
An alternative route to protein assembly at surfaces based on using the unique capabilities of biological materials for the spatially selective assembly of proteins is described. Specifically, the stimuli-responsive properties of amino-polysaccharide chitosan are combined with the molecular-recognition capabilities of biotin-streptavidin binding. Biotinylated chitosan retains its stimuli-responsive properties and is capable of electrodepositing at specific electrode addresses. Once deposited, it is capable of binding streptavidin, which can mediate the subsequent assembly of biotinylated proteins. Spatially selective protein assembly using biotinylated Protein A and fluorescently-labeled antibodies is demonstrated. (Macromol. Biosci. 2008, 8, 451.)
- Glucose Oxidase Mediated Gelation: A Simple Test to Detect Glucose in Food Products
- Biofabricating Multi-functional Soft Matter with Enzymes and Stimuli-Responsive
- Biofabrication: Programmable Assembly of Polysaccharide Hydrogels in Microfluidics as Biocompatible Scaffolds
- Electrodeposition of a Biopolymeric Hydrogel: Potential for One-step Protein Electroaddressing
- Electroaddressing Functionalized Polysaccharides as Model Biofilms for Interrogating Cell Signaling
- Redox Capacitor to Establish Bio-Device Redox-Connectivity
- Direct SERS detection of contaminants in a complex mixture: Rapid, single step screening for melamine in liquid infant formula
- Coupling Electrodeposition with Layer-by-Layer Assembly to Address Proteins within Microfluidic Channels
- Electroaddressing Agarose Using Fmoc-Phenylalanine as Temporary Scaffold
- Reversible Electroaddressing of Self-Assembling Amino Acid Conjugate
- Redox-Cycling and H2O2-Generation by Fabricated Catecholic Films in the Absence of Enzymes
- Biocompatible multi-address 3D cell assembly in microfluidic devices using spatially programmable gel formation
- In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes
- Engineered biological nanofactories trigger quorum sensing response in targeted bacteria
- Cross Species Quorum Quenching Using A Native AI-2 Processing Enzyme
- Magnetic nanofactories: Localized synthesis and delivery of quorum-sensing signaling molecule autoinducer-2 to bacterial cell surfaces
- AI-2 biosynthesis module in a magnetic nanofactory alters bacterial response via localized synthesis and delivery
- Biofabrication of antibodies and antigens via IgG-binding domain engineered with activatable pentatyrosine pro-tag
- Design optimization for bioMEMS studies of enzyme-controlled metabolic pathways
- Spatial resolution in chitosan-based programmable biomolecular scaffolds
- In situ generation of pH gradients in microfluidic devices for biofabrication of freestanding, semi-permeable chitosan membranes
- A Cantilever Sensor with an Integrated Optical Readout for Detection of Enzymatically Produced Homocysteine
- An Optical MEMS Sensor Utilizing a Chitosan Film for Catechol Detection
- Mechano-transduction of DNA Hybridization and Dopamine Oxidation through Electrodeposited Chitosan Network
- Electroaddressing of Cell Populations by Co-Deposition with Calcium Alginate Hydrogels
- Biomimetic Sealant Based On Gelatin and Microbial Transglutaminase: An Initial In Vivo Investigation
- Chitosan-Coated Wires: Conferring Electrical Properties to Chitosan Fibers
- Orthogonal Enzymatic Reactions for the Assembly of Proteins at Electrode Addresses
- Reagentless Protein Assembly Triggered by Localized Electrical Signals
- Chitosan-Coated Electrodes for Bimodal Sensing: Selective Post-Electrode Film Reaction for Spectroelectrochemical Analysis
- Chitosan Fibers: Versatile Platform for Nickel-Mediated Protein Assembly
- Chitosan Biotinylation and Electrodeposition for Selective Protein Assembly